LABORATORIO DE INMUNOPATOLOGÍA
The main purpose of our research is to identify cellular and molecular defects associated to autoimmune disorders and determine the mechanisms through which they contribute to the development and/or perpetuation of disease. Based at the Department of Immunology and Rheumatology of the Salvador Zubirán National Institute of Medical Sciences and Nutrition in Mexico City, we investigate the biology that underlies clinical phenomena observed in patients with autoimmune diseases through the use of in vitro and in vivo systems with the ultimate goal of developing novel conceptual models that will guide the identification of biomarkers and original therapeutic strategies.
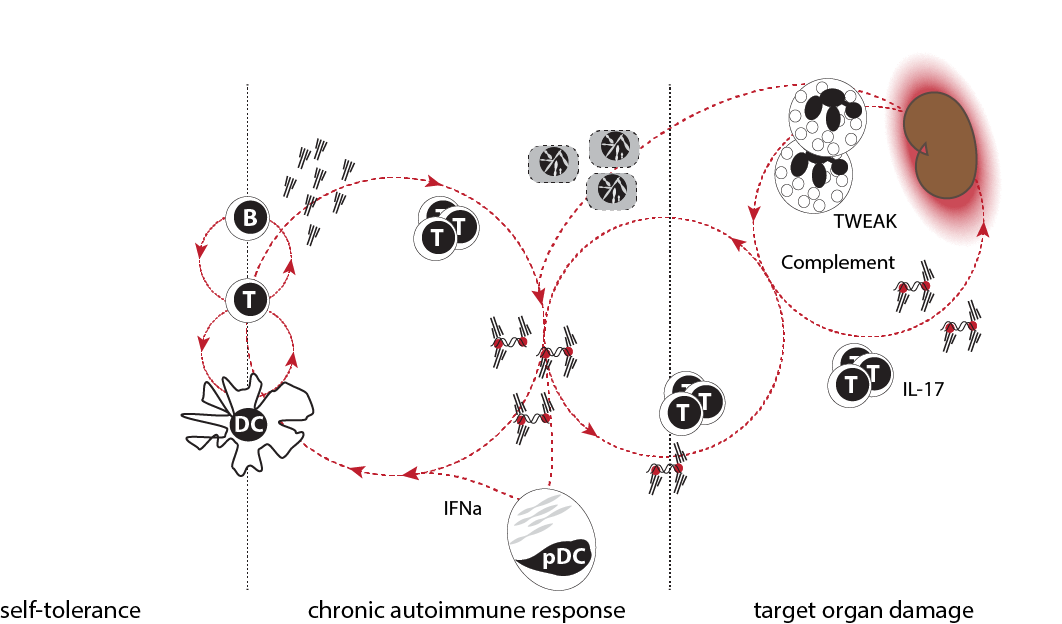
SLE pathogenesis in three phases. Immune responses are tightly controlled and loss of tolerance is transient in healthy humans and mice. Patients with SLE gradually develop a chronic autoimmune response characterized by constant production of auto-antibodies and low-grade immune activation. The clinical significance of this response is unknown. In some patients, products of the chronic autoimmune response cause target organ inflammation. The genetic and environmental triggers that promote loss of tolerance and initiation of organ inflammation are probably different. Likewise, the pathogenic pathways involved in phases two and three are undoubtedly distinct.
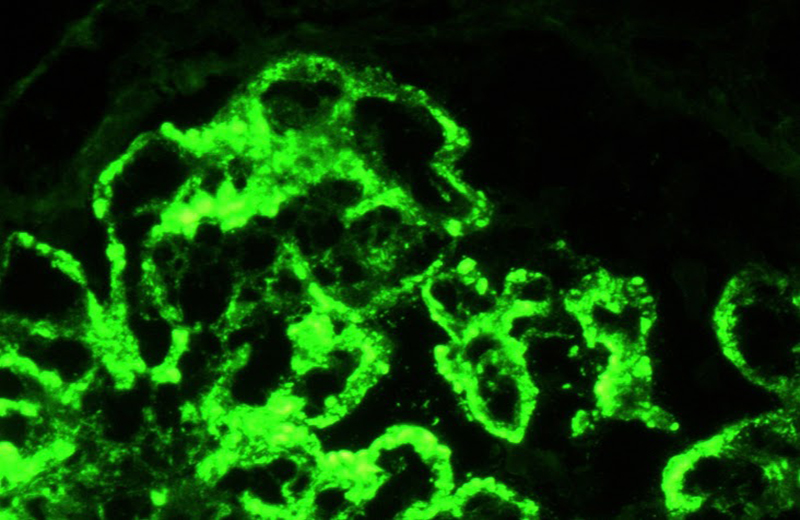
Type IV lupus nephritis.
Shown is a direct immunofluorescence against the light lamba chain.
Subendothelial, subepithelial and mesangial immune complexes are observed.
Photograph: Norma Uribe, MD (INCMNSZ)
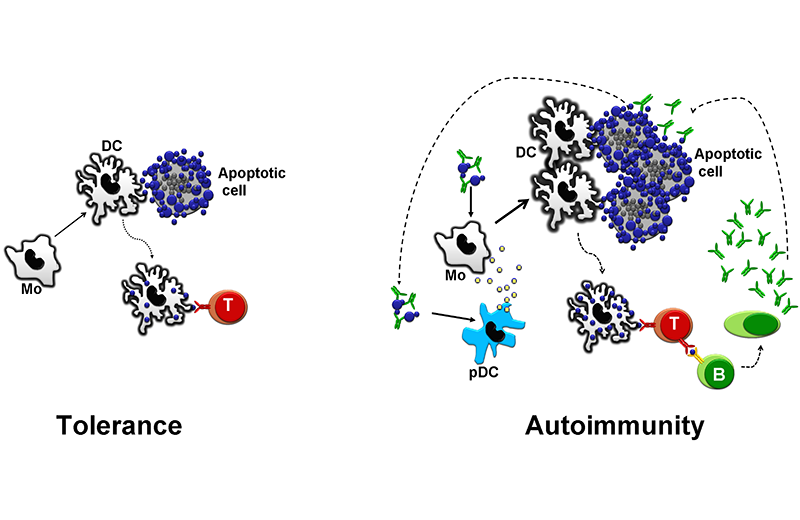
In healthy individuals, apoptotic cell debris are presented to T cells in a non-inflammatory context. This process maintains autoreactive T cells inactivated (left). Low-affinity Fc receptors, a low density of complement receptors, and genetic or acquired deficiency of complement factors diminish the capacity of the immune system of patients with lupus to scavenge immune complexes and apoptotic cell debris. These factors, along with an increased load of apoptotic cells, overload the immune system with autoantigens. The increased load in a susceptible individual leads to the development of autoantibodies. These autoantibodies opsonize apoptotic cells, which favors an inflammatory manifestation. Additionally, they form immune complexes that stimulate antigen-presenting cells, thus creating a vicious circle.







